確認某段製程透過特殊管制使製程結果(產品、服務、或其他的輸出)可以得到保障以持續符合預期要求。
提要 Summary
許多注重品質及品質系統的醫療器材公司, 已開始進行及實施製程確效的作業。透過製程確效流程不僅能掌握醫療器材產品品質, 並可減少全檢的負擔。
如何辨別需確效的製程, 並透過說明充分熟悉製程確效的概念及其做法,進一步為公司帶來品質及品質系統運作之效益。
大綱
1.Terminology, standard and regulations 術語、標準和法規
製程確效是在醫療器材常用到的名詞,大意是:
製程確效建立客觀的證據來確認製程,使用特別的管制方法使製程結果(成品,半成品或其他的輸出)可以達到持續穩定,並符合預期的要求。
Process validation means establishing by objective evidence that a process consistently produces a result or product meeting its predetermined specifications.
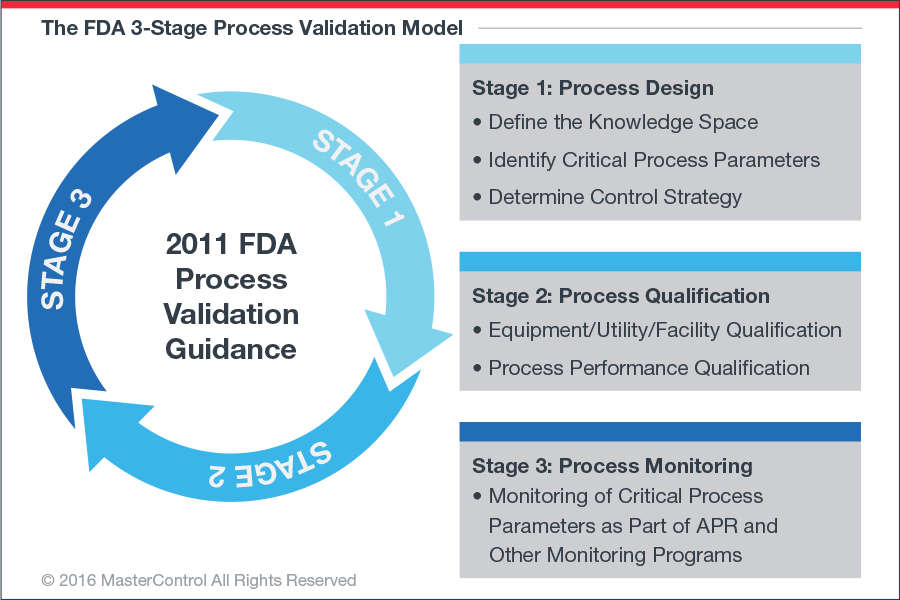
製程確效一般包含「計畫建立、搜集資料、解讀資料」的流程,流程一般分為以下三階段(稱為 3Q):
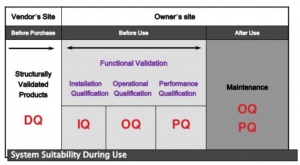
IQ (Installation qualification) 安裝驗證
通過客觀證據證明過程設備和輔助系統安裝的所有參數方面都符合製造商批准的規範,並且適當使用設備供應商的建議。目的:設備正確
Establishing by objective evidence that all key aspects of the process equipment and ancillary system installation adhere to the manufacturer’s approved specification and that the recommendations of the supplier of the equipment are suitably considered.
GHTF/SG3/N99-10:2004 (Edition 2), Definitions 2.1
OQ (Operational qualification)操作驗證
通過客觀證據, 建立過程控制的範圍(參數上下限),確定CAPA矯正預防行動的時機,從而使產品滿足所有預定要求。目的:參數範圍正確
Establishing by objective evidence process control limits and action levels which result in product that meets all predetermined requirements.
GHTF/SG3/N99-10:2004 (Edition 2), Definitions 2.2
PQ (Performance qualification)性能驗證
通過客觀證據確定該過程在預期條件下始終如一地生產出滿足所有預定要求的產品。
Establishing by objective evidence that the process, under
anticipated conditions, consistently produces a product which meets all predetermined requirements.
GHTF/SG3/N99-10:2004 (Edition 2), Definitions 2.3
Process validation protocol:
說明如何進行驗證的文件,包括測試參數、產品特性、製造設備以及什麼是構成可接受的測試結果。
A document stating how validation will be conducted, including test parameters, product characteristics, manufacturing equipment, and decision points on what constitutes acceptable test results.
GHTF/SG3/N99-10:2004 (Edition 2), Definitions 2.5
Verification:
通過檢查和提供客觀證據來確認已滿足規定的要求。
Confirmation by examination and provision of objective evidence that the
specified requirements have been fulfilled.
GHTF/SG3/N99-10:2004 (Edition 2), Definitions 2.6
2.When is process validation required? 何時需要製程確效
品質系統中的制程確效
制程確效是質量管理體係綜合要求的一部分。 它是在包括設計和開發控制、質量保證、過程控制以及糾正和預防措施在內的系統環境中進行的。
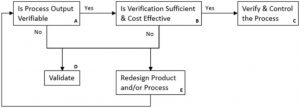
確認製程是否需要確效可參考以下的決策樹 (Decision tree):
這裏說明一下Validation 和Verification 的共同點與區別點:
共同點:確保製程可以穩定,並達到原先設定的品質或要求。
不同點:
Validation:瞭解製程的變異,確定在什麽變異範圍可以生產出符合標準的產品,並想辦法控制變異在可接受的範圍,來確保符合預期的輸出結果。
Verification: 利用及時性的品保措施如測試,檢驗和量測來確保製程輸出符合預期,如果后續仍發現製程的缺陷則可以考慮Validation。
FDA 以及ISO3485 對兩個方法的解釋
(a) Where the results of a process cannot be fully verified by subsequent inspection and test, the process shall be validated with a high degree of assurance and approved according to established procedures. The validation activities and results, including the date and signature of the individual(s) approving the validation and where appropriate the major equipment validated, shall be documented.
(b) Each manufacturer shall establish and maintain procedures for monitoring and control of process parameters for validated processes to ensure that the specified requirements continue to be met.
The organization shall validate any processes for production and service provision where the resulting output cannot be or is not verified by subsequent monitoring or measurement and, as a consequence, deficiencies become apparent only after the product is in use or the service has been delivered.
ISO 13485:2016, clause 7.5.6
3.Different types of process validation 不同的製程類型
如果鑑別出某些製程「無法由其的產品檢驗與測試被完全查證 (Verification),或製造產生的缺失只能在產品使用時被發現」,則需要進行製程確效 (Process Validation) 或重新設計產品或製程;如果可以由後續檢驗檢查出製造缺失時,則需要考慮這樣的檢驗是否足夠 ,是否符合成本,以下列舉「無法由隨後的產品檢驗與測試被完全查證 」一些例子:
- Sterilization processes 滅菌流程
- Clean room ambient conditions 無塵室環境
- Sterile packaging sealing processes 無菌密封包裝流程
- Lyophilization process 凍乾流程
- Heat treating processes 熱處理流程
- Plating processes 電鍍流程
- Plastic injection molding processes 射出成型流程
如果某製程可經由其產品檢驗與測試被完全查證,且「符合成本」,則須將相關的檢驗管制規劃到製程控制計畫中 。
4.An introduction to process capability studies 製程能力研究
5.Process validation protocols (IQ, OQ and PQ) 製程確效計畫書
6.Monitoring the state of process validation 監督製程確效狀態
7.Process revalidation 製程重新確效

Recent Comments